本帖最后由 老马 于 2012-6-4 00:04 编辑
A phase I dose escalation study of oral MK-2206 (allosteric Akt inhibitor) with oral selumetinib (AZD6244; ARRY-142866) (MEK 1/2 inhibitor) in patients with advanced or metastatic solid tumors.
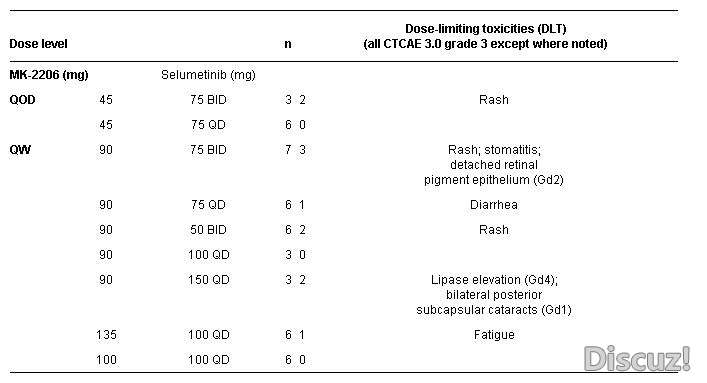
Background: Simultaneous inhibition of both the PI3K-Akt and RAF/MEK/ERK pathways may yield greater benefits than inhibiting either pathway alone. This phase I study (NCT01021748) examined the safety, pharmacokinetics (PK), pharmacodynamics (PD), maximal tolerated dose (MTD), and preliminary antitumor activity of the combination of a MEKi (selumetinib) and AKTi (MK-2206). Methods: Eligible patients (pts) with advanced solid tumors were treated with MK-2206 either every other day (QOD) or once weekly (QW), in combination with selumetinib administered either once daily (QD) or twice daily (BID). Results: 51 pts with advanced solid tumors (15 colon, 8 NSCLC, 6 ovarian, 5 pancreatic, 3 breast and 14 others) were treated across 9 dose levels. There were 2 confirmed partial response (PR) (1 NSCLC, ongoing > 52 wks; 1 ovarian, on treatment for 47 weeks; both KRAS mutation positive), 1 unconfirmed PR (pancreatic, on treatment 20 wks), and 24 pts with stable disease (ranged from 6 to 47 wks). Preliminary assessment of PK data suggested no apparent drug-drug interactions with unaltered PK profiles of each drug when administered in combination. Conclusions: In combination the maximum tolerated doses of MK-2206 and selumetinib are 135 mg QW and 100 mg QD, respectively. This combination of investigational agents demonstrated preliminary antitumor activity in pts with advanced cancer.
http://abstract.asco.org/AbstView_114_99901.html
|